Prepared by Nguyen Danh Thien – PhD student at USTH
Introduction
Up to date, plastic is one of the most useful inventions in the world. However, plastic wastes has become a serious environmental problem in developing countries including Vietnam (Lahens et al., 2018). Microplastic which are fragment and segment of plastic broken down into smaller size of 1 – 5000 μm (Boyle and Örmeci, 2020,Mammo et al., 2020). The existence of microplastic in aquatic environments can lead to accumulate in the whole trophic network because they can be kept in the digestive systems of organisms and be transported to other organs/tissues depending on size, shape, and polymer types (Puckowski et al., 2021). Several studies demonstrated that MPs can act as a vector transport of organic pollutants such as antibiotic residues which has been reported in aquatic environments (Du et al., 2024; Atugoda et al., 2021, Binh et al., 2018). However, the study of the combination of polypropylene (PP) and/or polylactic acid polymer (PLA) with sulfamethoxazole (SMX) and oxytetracycline (OTC) is still limited information. Thus, the ecotoxicological tests were conducted to assess the toxicity of mixing PP and PLA with antibiotics for bivalve species.
Experiment design
Freshwater pearl mussels Hyriopsis cumingii and Pacific oysters Crassostrea gigas commercial were chosen for the ecotoxicity experiment because of their value in both ecology and economics. Before performing the experiments, oysters and mussels were assimilated for seven days (Figure 1 and 2). PP and PLA with size < 200 μm have been used at a concentration of 10 mg/L. SMX and OTC have been chosen due to their wide application in aquaculture activities in Viet Nam and based on the concentrations found in the environment at 30 μg /L for SMX and 20 μg/L for OTC in freshwater, and 64 ng/L SMX in marine water.
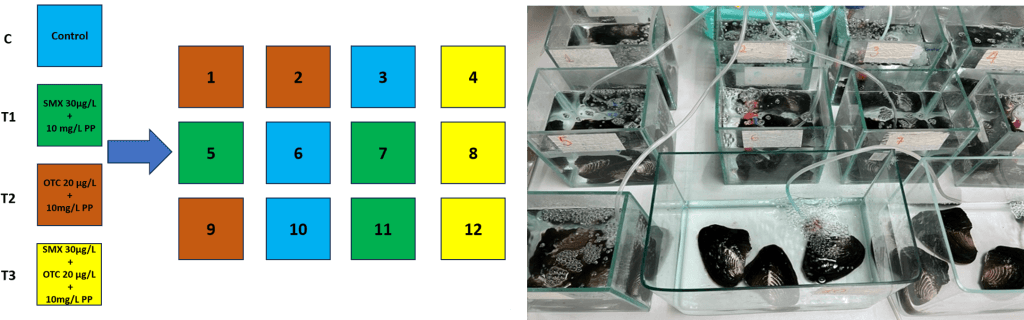
Figure 1: Impact of polypropylene (PP) microplastic associated with sulfathoxazole (SMX) and oxytetracylene (OTC) on freshwater pearl mussels
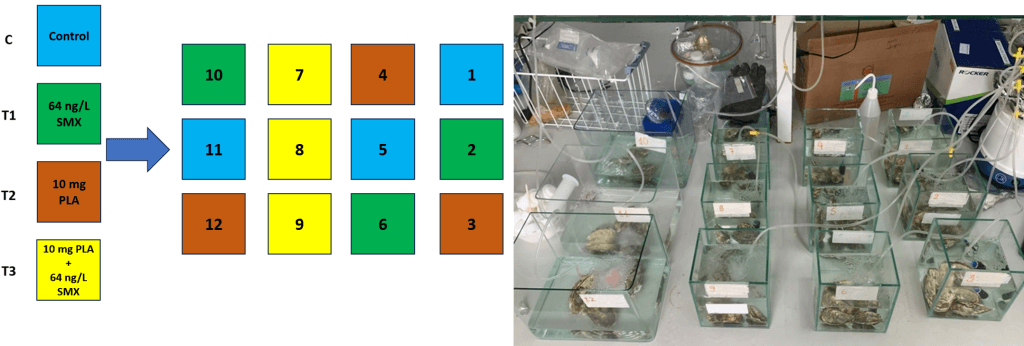
Figure 2: Impact of polylactic acid polymer (PLA) microplastic and sulfathoxazole (SMX) on Pacific oyster
Microplastic analysis
To extract the concentration of MPs inside Pacific oysters, we use the method of extraction of MPs based on the method of (Zhou et al., 2020) (Figure 3). Separated samples were observed by the Leica stereomicroscope S9i to identify the presence of PP and PLA in the gills and intestine tissues and using µFTIR instrument to identify PP and PLA (Nicolet In10Mx Dual Director microscope, Thermo Fisher Scientific).
Bioassay analysis
Cellular extraction from tissues
Firstly, we used MEM solution contained collagenase enzyme to dissociate cells from gill and intestine tissues of oysters and mussels. Then we checked cell viability in all samples and the percentage of cell viability was determined constantly above 90 % under Leica inverted microscope DM 2500 LED for further analysis.
Comet Assay
Comet assay has been followed by protocol of RnDSystems with catalog number 4252-040-K. All slices were observed under Leica fluorescence microscopy DMIL LED Fluo and lecturing by Trevigen Comet Analyzer V1.3d for analyzing the DNA damage (Figure 3).
Micronucleus assay
The method to determine the micronucleus was referred from study Le Bihanic et al. (2014). The slice was observed using a Leica fluorescence microscopy DMIL LED Fluo after dying with acridine orange 0.003% (Figure 3).
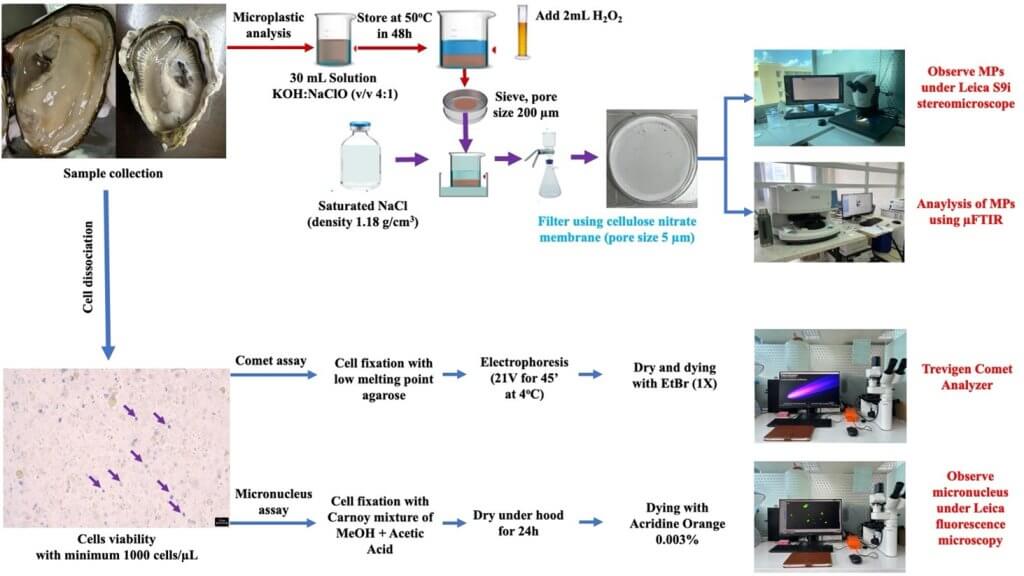
Figure 3: Microplastic analysis and bioassay analysis
Primary result
In these studies, PP and PLA microplastic were detected in the gills and intestine tissues of Pacific oysters and freshwater mussels (Figure 4). The results also found more genotoxicity effects due to core badly damaged for mussels and oysters exposed to PP or PLA microplatics in combination with antibiotics SMX and/or OTC (Figure 5 & 6). Interestingly, mussels exposed to PP microplatics associated with only OTC or with OTC mixed with SMX showed more DNA damage than those exposed to PP microplatics associated with only SMX. However, further study is necessary to find the mechanism of genotoxicity effects for those contaminants in the future.
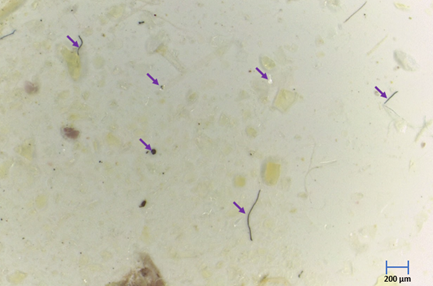
Figure 4: MPs observation under Leica stereomicroscope S9i
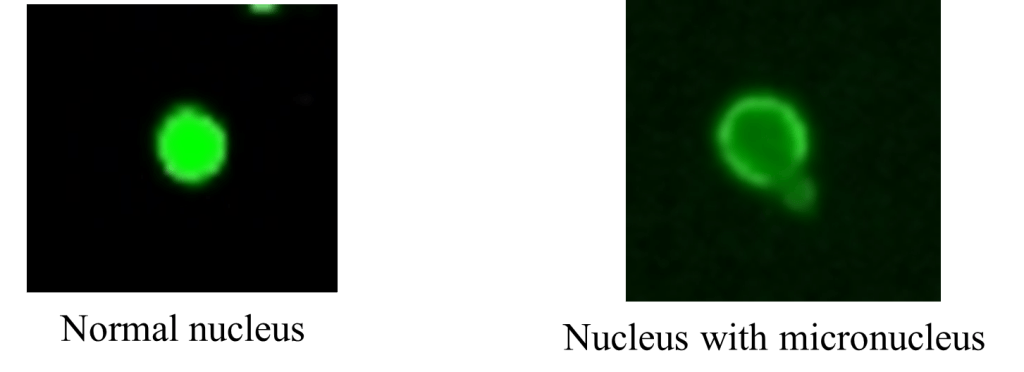
Figure 5: Micronucleus observation under fluorescence microscope
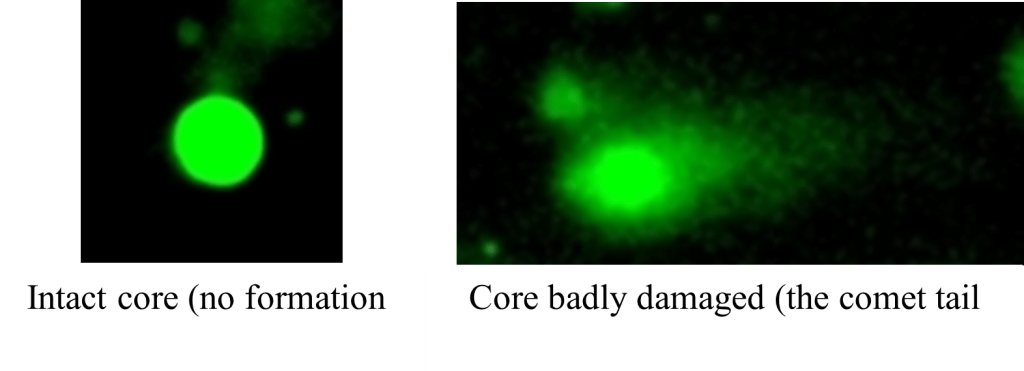
Figure 6: DNA fragment observation under the fluorescence microscope
Reference
- Atugoda, T. et al. (2021) ‘Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport’, Environment International, 149, p. 106367. Available at: https://doi.org/10.1016/j.envint.2020.106367.
- Binh, V.N. et al. (2018) ‘Antibiotics in the aquatic environment of Vietnam: Sources, concentrations, risk and control strategy’, Chemosphere, 197, pp. 438–450. Available at: https://doi.org/10.1016/j.chemosphere.2018.01.061.
- Boyle, K. and Örmeci, B. (2020) ‘Microplastics and Nanoplastics in the Freshwater and Terrestrial Environment: A Review’, Water, 12(9), p. 2633. Available at: https://doi.org/10.3390/w12092633.
- Du, J. et al. (2024) ‘Ecotoxicological Effects of Microplastics Combined With Antibiotics in the Aquatic Environment: Recent Developments and Prospects’, Environmental Toxicology and Chemistry, p. etc.5950. Available at: https://doi.org/10.1002/etc.5950.
- Lahens, L. et al. (2018) ‘Macroplastic and microplastic contamination assessment of a tropical river (Saigon River, Vietnam) transversed by a developing megacity’, Environmental Pollution, 236, pp. 661–671. Available at: https://doi.org/10.1016/j.envpol.2018.02.005.
- Le Bihanic, F. et al. (2014) ‘Developmental toxicity of PAH mixtures in fish early life stages. Part II: adverse effects in Japanese medaka’, Environmental Science and Pollution Research, 21(24), pp. 13732–13743. Available at: https://doi.org/10.1007/s11356-014-2676-3.
- Mammo, F.K. et al. (2020) ‘Microplastics in the environment: Interactions with microbes and chemical contaminants’, Science of The Total Environment, 743, p. 140518. Available at: https://doi.org/10.1016/j.scitotenv.2020.140518.
- Puckowski, A. et al. (2021) ‘Sorption of pharmaceuticals on the surface of microplastics’, Chemosphere, 263, p. 127976. Available at: https://doi.org/10.1016/j.chemosphere.2020.127976.
- Zhou, Y., Liu, X. and Wang, J. (2020) ‘Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida’, Journal of Hazardous Materials, 392, p. 122273. Available at: https://doi.org/10.1016/j.jhazmat.2020.122273.